Taking a closer look into fibroblasts in the central nervous system
The central nervous system (CNS) consists of several types of cells having varied specialized functions. While many of these cell types have been studied, fibroblast cells in the CNS remain underexplored. Commonly found in connective tissue, fibroblasts produce proteins that maintain the structural framework of tissues and organs. Additionally, they play an important role in healing wounds, fighting diseases, and homeostasis.
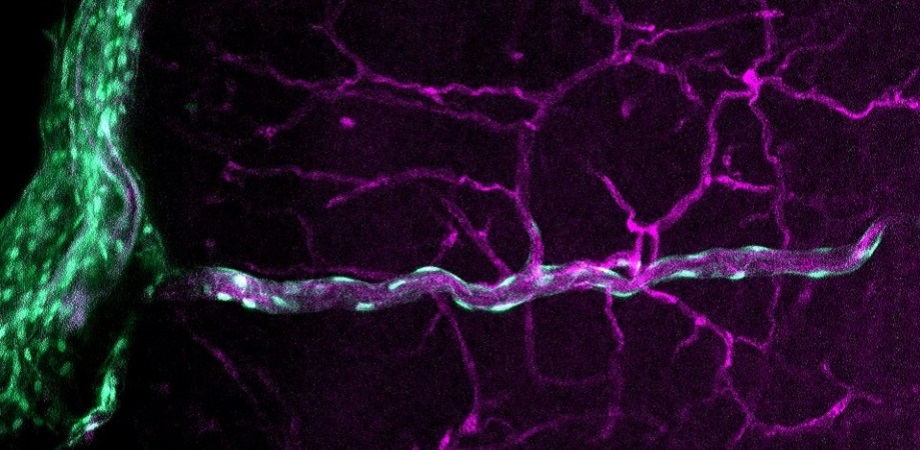
CNS fibroblasts are found in the meninges (protective membranes surrounding the CNS), the connective tissue of the choroid plexus (which produces the cerebrospinal fluid), and along the vascular system in the brain parenchyma (functional tissue of the brain). Little is known about how CNS fibroblasts originate in the body and enter in the CNS, their functions in the CNS, and their interaction with other CNS cell types. This is because current visualization methods are inadequate for a complete assessment of CNS fibroblasts.
To address this issue, a group of researchers led by Associate Professor Julie Siegenthaler from the University of Colorado, USA, developed new method for better visualizing the CNS fibroblasts and their intercellular interactions within the CNS. Explaining the purpose behind their study, Siegenthaler says, “Fibroblasts in non-CNS tissues and organs play a vital role in homeostasis and fighting illnesses. Since very limited research exists on CNS fibroblasts, we wanted to give particular attention to these cells so as to understand their developmental origins and tissue-specific functions within the CNS.”
In their report published in Neurophotonics, the team provides a detailed description of their technique, which includes dissection, immunohistochemistry (antibody-based method to identify antigen proteins in tissue samples), whole mounting, and visualizing CNS tissues in the meninges and choroid plexus during different developmental stages (embryo, newborn, and adult).
The work adapts and optimizes the “CUBIC method” for optical tissue clearing (making tissues transparent) to help visualize and examine fibroblasts present in the brain parenchyma during early stages of birth and adulthood. Short for “clear, unobstructed brain (or body) imaging cocktails and computational analysis,” CUBIC facilitates study of large biological samples using microscopy. The method was tested and optimized on mice models genetically modified with green fluorescent protein for easy cell/tissue labeling and identification.
The techniques devised by Siegenthaler’s team are expected to provide significantly better results as compared to the conventional techniques used to study CNS tissues and cells. “Our whole mount method will enable identification of different subpopulations of CNS fibroblasts and study their cellular origins and molecular identity. It will also be beneficial in closely investigating their role in development, health, aging, disease and regulating immune cell activity,” says Siegenthaler.
The team was able to visualize fibroblasts in the meninges and choroid plexus, and their interactions with other cells and blood vessels. Their technique not only produced images with a more comprehensive picture of the CNS fibroblasts but did so at different focal distances, allowing these images to be composed for a 3D rendering of the structures.
The images obtained can be used to conduct in-depth studies on the location, size, morphology, intercellular interactions, and patterns of gene and protein expression of CNS fibroblasts. They can also be used in conjunction with other techniques to study and label different cell types found in the CNS and their interactions during tissue development and regeneration. Regarding the potential outcomes of their study, Siegenthaler remarks, “We expect our methods to create new opportunities for the investigation of CNS fibroblasts. More importantly, they could potentially boost neurological research by improving our understanding of how CNS fibroblasts maintain the health and homeostasis of the CNS.”
Read the open access article by Jones, Abrams, and Siegenthaler, “Techniques for visualizing fibroblast-vessel interactions in the developing and adult CNS,” Neurophotonics 9(2) 021911 (2022), doi 10.1117/1.NPh.9.2.021911.
| Enjoy this article? Get similar news in your inbox |
|



