LED Array Implant Illuminates the Future for Optogenetics Studies
Using optogenetics, scientists can selectively activate or deactivate neurons by illuminating them with light, allowing researchers to perform fine-grained cause-and-effect studies between brain activity and behavior. Most studies are currently performed in mice and other small mammals, because their small brains can be accessed with just a few optic fibers.
However, if larger mammals are to be studied using this method, then new techniques to illuminate specific brain regions and even specific clusters of neurons need to be developed. The challenge is to overcome the problems of brain size, light scattering, and the undesirability of overheating brain tissue, while still allowing the subject the freedom to move around without being tethered to a light source or a computer.
The brain is a scattering medium: every vesicle, cell membrane, and fluid deflects the path of the light. The problem is further exacerbated by the fact that hemoglobin strongly absorbs blue light, which is the color most often used in optogenetics. Because of these obstacles, the light source must be quite close to the target to operate with any precision.
To address these challenges, a team of researchers from Universities of Strathclyde and Utah have developed an LED optical array that is capable of independently illuminating specific brain regions, while also providing wide-area (4 × 4 mm) brain surface illumination. And if operated correctly, everything stays cool.

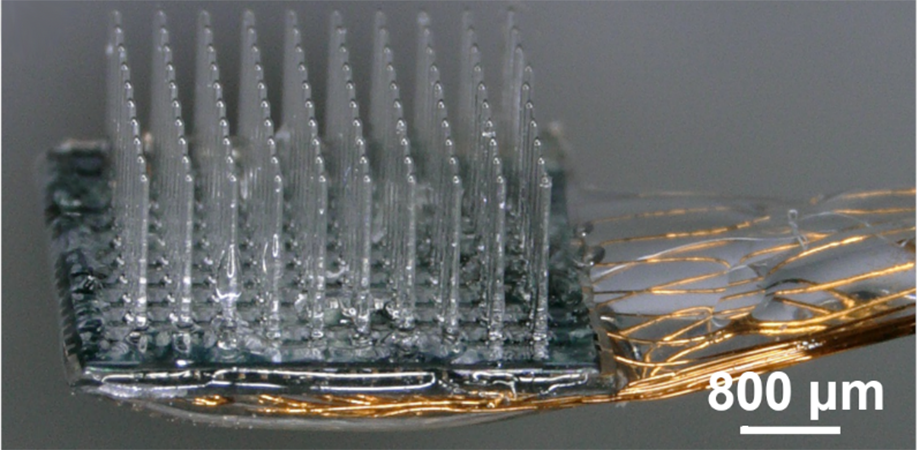
(a) Schematic of the integrated device with the glass UOA bonded to a microLED array. Illumination is from the microLED, through the sapphire (which is bonded to the glass UOA) and delivered to tissue either by the glass needles or through interstitial sites. A pinhole layer (not shown) is patterned onto the sapphire substrate of the microLED array before bonding to reduce optical crosstalk. (b) Completed device with polymer-coated wire bonds permitting independent control of the microLEDs. (c) Illumination of a single microLED delivering high-intensity light to the needle tip. (d) All 181 sites simultaneously illuminated.
Battery-powered LEDs
The device consists of a square 10 × 10 array of glass needles. An array of tiny light-emitting diodes (called µLEDs) are then aligned over the needles. This results in 100 glass needles that guide light from an individual LED to a depth of 1.5 mm in tissue. An additional 9 × 9 array (81 µLEDs) was placed in between the needles, allowing large-volume illumination as well.
However, even with careful placement, the team found that very little of the LED light makes it into the needles. This has two consequences: it means that the light exiting the needle is pretty weak and won't be able to control many neurons without using higher power, and also that the leaked light will activate neurons near the brain surface at undesired moments.
To overcome the first problem, the research team investigated the coupling between the light source and the waveguide. They discovered that the main problem is that the LEDs and the glass needles were too far apart (about 300 µm). In the current iteration of the array, the spacing was fixed due to the substrate on which the glass needles are produced and the substrate that holds the LEDs. Nevertheless, the researchers found that the current configuration delivered sufficient optical power to the end of the needle to impact up to 5500 neurons per needle.
The bigger problem of the poor coupling between the LEDs and the needles was the stray light emitted from the substrate into the brain surface. To solve this problem, the researchers simply placed a pinhole mask over the substrate so that light could only be emitted from the needles and the surface LEDs.
With all tweaks in place, the researchers showed that they had a good controllable light source that could, in principle, provide pinpoint control via the needles, as well as large-volume activation from the surface LEDs.
In the future, the group will modify the fabrication procedure. By replacing the glass substrate with a thinner silicon substrate, the coupling efficiency can be improved. And, because silicon naturally absorbs blue light, the substrate will also reduce stray light.
Keeping it cool
The team also modeled the thermal behavior of the array to estimate the temperature it would reach. To obtain sufficient light intensity to control a large number of neurons, the LEDs had to be operated at quite high power, and when they were all switched on continuously, the array heated up by some 60°C, which was unacceptable. To prevent heating brain tissue above 1°C under intense activation conditions, the LEDs must be operated with a duty cycle of to one to two percent—a limitation that could be improved by using a silicon substrate with better coupling.
Even with a limited duty cycle, this device hints at an even brighter future for optogenetics. It delivers precisely controllable light via the needles, while also providing wide area illumination. The entire array can be battery powered and implanted using standard techniques. The authors state that, with only minor modification, the LED drivers can be controlled wirelessly, meaning that the subject could have full mobility.
Read the original article in Neurophotonics: N. McAlinden, "Multisite microLED optrode array for neural interfacing," Neurophoton. 6(3), 035010 (2019).
Related SPIE content:
Optogenetics: Illuminating Our Head Space
Neurophotonic strategies for observing and controlling neural circuits
| Enjoy this article? Get similar news in your inbox |
|



