Multiphoton Microscopy: Deeper, Wider, and Faster
The remarkable capabilities of optical microscopes that can obtain high-resolution mouse brain images directly through the surrounding skull promise to transform how we understand ourselves. Chris Xu, whose team at Cornell University, in Ithaca, US developed the technique, explains that the capability arises from multiple photons simultaneously interacting with molecules in the brain. Together they excite and make the molecules fluoresce. Each photon can therefore be lower energy, and therefore longer wavelength, than if a single photon was causing the fluorescence. "This allows you to penetrate better," Xu explains. "This is the same logic why the sky is blue, and sunset is red, right? Long wavelengths scatter less, including in tissue and bone."
Penetrating skulls is therefore just one of the many breathtaking capabilities two- and three-photon techniques offer, steadily driving their adoption. In fact, multiphoton microscopy can be applied even without adding colored or fluorescent molecules to enable cells to be imaged like many other approaches do, Xu explains. Instead, intense infrared multiphoton excitation makes molecules fluoresce that are naturally found in cells, like the key metabolic cofactor NADH. Xu and other pioneers in the field announced the latest frontiers such methods have reached at SPIE Photonics West 2020.
The most commonly used technique is two-photon excited fluorescence. The probability of two photons reaching a molecule and being absorbed by it to produce excitation is low, and therefore the fluorescence signal is relatively weak, Xu explains. "To make it practical for biological applications, a femtosecond laser must be used," he says. "You bunch photons in time so when the laser is on, so it's extremely bright. By doing so you can enhance this nonlinear excitation probability." Other approaches exploit scattering rather than absorption, for example second harmonic generation analysis of collagen, which has been used in cancer diagnostics, Xu notes, and third harmonic generation, which is useful for looking at myelin in the brain.
Multiphoton microscopy is a powerful tool for high resolution imaging in a 3D sample that is optically opaque, echoes Na Ji from the University of California, Berkeley, US. This includes most biological tissues. "The fluorescence signal is only generated at the focal spot of the microscope due to the very small cross-section of multiphoton excitation," she says. "As a result you just need to focus your light at a particular position in the sample and collect all the fluorescence photons coming out." To generate a large image involves scanning the laser focus at very high speed across the sample and record the fluorescence brightness at each position.
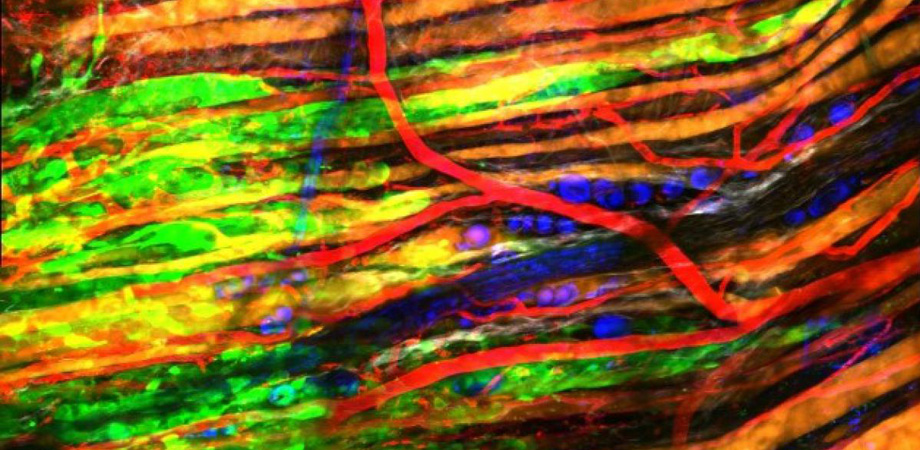
Na Ji's team uses multiphoton microscopy to image neurons from L2/3 to L6 of the primary visual cortex in the brain of a mouse. Credit: Na Ji/University of California, Berkeley.
Darryl McCoy from Santa Clara, US, headquartered Coherent Inc. stresses that restriction of absorption to the focal point is only practical with femtosecond lasers. Femtosecond technology also has advantages beyond providing high peak powers that enable multiphoton absorption in relevant fluorescent probes, McCoy adds. Femtosecond sources also "provide that peak power without raising the average heat output, preventing thermal damage to biological samples," he says. The long wavelengths used also help further reduce scattering, enabling penetration even deeper into tissue.
Putting disease in the picture
McCoy's colleague Marco Arrigoni adds that the largest consumer of multiphoton microscopy is neuroscience, due to the relatively large amount of funding available. "The brain is actually a friendly organ to look at, because the tissue of the brain is more transparent," he says. And three-photon microscopy is pushing brain studies further, going 1.5-2 times as deeply as two-photon approaches, he adds. Such capabilities are enabling potential future directions, such as improving or replacing current histopathology methods to tell whether tissue is cancerous using multiphoton microscopy together with computer algorithms. Arrigoni also raises the potential that multiphoton microscopes could be used in surgery. "People would look with multiphoton microscopes at the tissue being operated on, for example, for brain cancer," he says.
Michelle Digman, from the University of California, Irvine, US takes multiphoton microscopy in yet another direction: making it more accessible to non-experts. She highlights that multiphoton microscopy is interesting in many biologists without deep knowledge of its technical basics, because it excites autofluorescent components without exposing samples to harmful UV light. Exploiting how long the fluorescence signal or photon from two-photon excitation microscopy is emitted in fluorescence lifetime imaging microscopy (FLIM) is a powerful way to investigate complex biological systems
non-invasively, Digman says.
Building on this, Digman and her University of California, Irvine colleague Enrico Gratton co-developed phasor-FLIM analysis. This can identify fluorescence lifetime signatures from multiple molecule types in an image, she explains. Conventional FLIM uses fitting techniques that identify the different molecules by the extent to which two-photon excited autofluorescence signatures in biological samples decay. By transforming the histogram of time of arrival of photons into a polar coordinate system, Digman does away with the need for fitting. Because phasor-FLIM is a fit-free method, it does not require an expert to analyze the image, which makes it more accessible and more robust to observer-induced biases or artefacts. It is also easier to calculate the fraction of different fluorophores in any given pixel of an image.
Researchers are therefore using this approach to study metabolism in various parts of the body such as the liver, kidneys and intestines. "It's becoming a very useful way to both characterize cells or tissues in response to drugs, or in diseased cells," Digman says. "We've looked at Huntington's disease, and we've seen cells that have actually aggregated this protein called huntingtin, they form these plaques. They've actually have shifted the metabolism within the cell. Across the spectrum in neurons, in cancer biology — FLIM is a very useful tool to look at metabolism in almost any disease."
Digman's talk at Photonics West took place Sunday, February 2 in session 4 of Multiphoton Microscopy in the Biomedical Sciences XX. "The work we are presenting at Photonics West is using FLIM to determine what's happening in the development of embryos in a label-free, fit-free way. Now we have a way to characterize the signatures of healthy embryos at any embryonic stage," she says. This can be used to predict the best developmental potential in pre-implantation embryos for in-vitro fertilisation. Her team has also used two-photon microscopy to induce double-stranded DNA breaks. "We've been able to show that there is a pro-survival response to DNA repair activation through a change in cellular metabolism, which is proving to be critical in discovering new potential protein targets for treatment and for the understanding of genetic and carcinogenic consequences of diseases," Digman explains.
Pushing limits
Implementing the phasor approach is easy, asserts Digman, which microscope makers are aware of, and exploiting. Phasor-FLIM is therefore fairly widely used by groups she has never even talked to or heard of. "We're happy that other people are using it," Digman says. However she would like Phasor-FLIM to be far more widespread. Digman suggests that the software that groups use should be updated as improvements are consistently being made in identifying fluorophore lifetimes in mixtures. Currently the approach can distinguish two or three fluorophores. "The question is, can we start unmixing four, five, six fluorophore lifetimes that could be within that same pixel," Digman says. "We are now making efforts in that regard to unmix those possible lifetimes."
Na Ji is likewise pushing the limits to increase scanning speed for 3D imaging using multiphoton microscopy. By scanning long, thin Bessel beams through a sample in 2D, signals are generated across the length of the beam, she explains, which changes the 2D frame rate of microscope capture into a "3D volume rate." Samples being studied are kept perfectly still, Ji says. "We know where everything is exactly, we just need to look at how the signal changes with time," she adds, with these changes reflecting ongoing activity of the neurons in the brain. Newton, US-headquartered optical equipment company ThorLabs has licensed the approach from Ji's team to make add-on units for its commercial two-photon microscopes.
3D reconstruction of a labeled neuron located about 140 microns below the cortical surface in a mouse imaged by three-photon microscopy. Credit: Chris Xu/Cornell University
In session 2 of Multiphoton Microscopy in the Biomedical Sciences XX, Ji presented a collaboration with Kevin Tsia at the University of Hong Kong. Tsia has developed a "super-fast way of scanning a focal point in one dimension, so you can scan eight million lines per second," Ji says. "We can image samples at 3000 frames per second," she adds. They have used the work to image membrane potentials arising from electrical signals within the brain.
Yet data handling for this ultrafast raster scanning approach is a big challenge. A few seconds of imaging generates "a huge amount of data" Ji says. Ji and Tsia are collaborating with the Paninski lab at the statistics department at Columbia University, in New York, US, among others, to develop techniques to resolve the issues. "It will probably be five to ten years before you can see an easy-to-use commercial product for biologists," she says.
The approach is part of the three biggest trends that Ji sees in multiphoton microscopy: improvements in speed, resolution and depth of imaging. Speed is important because some of the biological processes being investigated happen on a millisecond scale, Ji says. "This requires kilohertz imaging, with speeds of one image per millisecond," she says. "The higher resolution part is where adaptive optics is, I believe, essential, to correct for sample-induced distortion of the excitation light. And Chris Xu has developed this three-photon excitation microscopy, which is really pushing the limit of how deep we can image."
In his talk, also in session 2 of Multiphoton Microscopy in the Biomedical Sciences XX, Xu presented in line with his slogan, "Imaging deeper, wider, and faster," he says. "For imaging deeper, we are pushing the three-photon, longer wavelength approach," Xu explains. "Wider imaging is to build large area imaging microscopes. For imaging faster, we are presenting a new laser source that we have developed that increases photon efficiency. We want to make sure every photon we put in the brain is doing work for us, not just heating up the brain."
Wide-ranging usefulness
Xu adds a further important trend beyond deep imaging of intact tissue, which is translation of the technology to biological and clinical applications. Deep imaging allows the imaging of biological processes in their natural environment, he stresses. "If you take a neuron out of the brain, you can look at it very carefully, but it's probably not very meaningful," Xu says. Yet this requires a laser, which is ultra-fast, with the right wavelength, pulse energy, and right repetition rate among other factors, Xu adds. That brings one of multiphoton microscopy's key challenges in the cost of lasers with these capabilities, which begin at $20,000 and go up to $500,000.
Coherent's McCoy says that serving a range of "who want huge amounts of flexibility and performance from the laser, to customers that are really focused on price and size' isn't straightforward. ‘We have to create a suite of femtosecond lasers that from the outside is very biologist friendly and yet on the inside integrate quite complicated laser techniques and technology,' he says. ‘In general that means that you have challenges in creating lasers that are small enough and cheap enough to really expand the market."
3D reconstruction of three-photon microscopy through-skull imaging of a cortical column of neurons in a mouse, with different proteins labeled red and green. Credit: Chris Xu/Cornell University
The two multi-photon products that Coherent presented at Photonics West are the Axon series of single wavelength femtosecond solutions, and the Chameleon Discovery NX. "The Axon series has been developed for the main popular fluorescent probes at 920nm and 1064nm, with other wavelengths on the way," comments McCoy. "The Chameleon Discovery NX offers industry-leading power, pulse width and modulation capability in a single one-box flexible platform. We also display lasers for advanced imaging techniques such as deep imaging using three-photon excitation, that employ high energy fiber-laser technology and optical parametric amplifiers."
Yet adoption of femtosecond lasers and multi-photon techniques outside universities is slow, Arrigoni adds. Replacement of conventional histology with lasers is still far away, due to the entrenchment of the incumbent technology. "Our part in this is to develop laser sources that are more compact, more reliable and more effective, like the Axon family," he says. "Most of the activity right now is academic research with longer-term horizon of addressing neurodegenerative diseases and some types of cancer."
Nevertheless, Xu highlights the importance of the relationship between academic groups and laser producers in bringing multiphoton microscopy to the mainstream. "When we started three-photon imaging about seven, eight years ago, we worked with commercial companies," he says. "Now these lasers are commercially available from a minimum of six companies. They're actually pretty fast coming online."
Xu adds that he feels this synergistic relationship will continue, "because it's very fruitful so far for all of us. It is estimated that there are 4,000-8,000 two-photon microscopes across the world, so it's not a niche anymore," he says. "For the three photon imaging techniques, 40-50 groups are using it, it's remarkable really given that lasers were commercially available only a few years ago. Most of the technologies developing in the multiphoton field are reasonably translatable. You can buy a multiphoton microscope, do your favorite experiments, and write great science with it. It's interesting — this whole field has only taken 30 years from initial demo to many people doing imaging with this technology."
Andy Extance is a freelance science journalist based in the UK. A version of this article was originally published in the SPIE Photonics West Show Daily.
Related SPIE content:
Plumbing the Depths of Multiphoton Microscopy
Multiphoton microscopy: a personal historical review, with some future predictions
Faster, wider, deeper: imaging advances in focus at Hot Topics session
Recent advances in lasers for multiphoton microscopy (Conference Presentation)
Na Ji: Adaptive optics enables new optical microscopy for neuro applications
Chris Xu: 3-photon microscopy for deep brain imaging
| Enjoy this article? Get similar news in your inbox |
|






