From Research to Commercialization: AI Diagnostic Tool Aims to Improve Breast Cancer Diagnosis
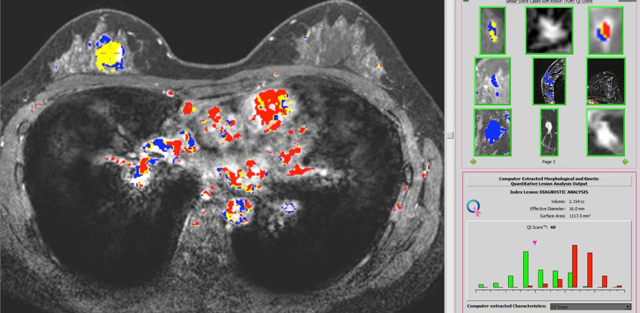
In 2017, a computer-aided, breast-cancer diagnosis system, QuantX — which combines clinical imaging techniques with innovative AI-driven software — became the first FDA-cleared machine-learning driven system to aid in cancer diagnosis for use in radiology. QuantX grew from the research of SPIE Fellow and Past President Maryellen Giger and her team at the University of Chicago, and was incubated at Quantitative Imaging, a University of Chicago startup. QuantX was recently acquired by Qlarity Imaging, a portfolio company of Paragon Biosciences.
An innovative imaging success story? Absolutely! But the paragraph above belies the years of research, testing, and collaboration that led to a successful start-up being purchased by an industry powerhouse. In fact, the road from bench to bedside is one marked by an often grueling, long-term history of persistence, patience, and due diligence.
From lab algorithm to medical startup
Giger has been working on computer-aided detection since the mid-80s, developing methods to extract information from images using computers. "At that time, we were trying to detect lesions in mammograms and lung nodules in chest radiographs," says Giger. "Those developments have already been translated: in the US, approximately 90 percent of screening mammograms include some element of computer-aided detection as an aid to the interpreting radiologist." The next step was developing additional AI methodologies to enhance and speed-up diagnosis: that is, given a lesion, what is the likelihood that it is cancer or not? What further information was needed so that a radiologist could recommend the next, best patient-management step?
"That is the most recent translation," says Giger. "For images of breast lesions, such as on mammograms, ultrasounds, or MRIs, we developed new analytics and new software — that is, based on the biomedical understanding of the cancer as well as the physics of the image acquisition system, we develop the mathematics behind the classification task." To be successful in this area, Giger continues, you need an interdisciplinary team to understand a range of biomedical and technical questions: What is the task? Are you conducting detection? Are you performing diagnosis? Are you assessing response to therapy? How can you rigorously evaluate the software so that it will be clinically useful?
It is interesting to note that once you have developed laboratory-validated software for medical image interpretation, there are still numerous logistical and institutional processes that need to be addressed prior to introduction to routine clinical use. In the case of QuantX, the process started during the 2009-10 academic year when two MBA students, a PhD graduate student in Giger's lab, and a medical student, all from the University of Chicago, put a team together and took the Breast MRI Computer-Aided Diagnosis workstation through the New Venture Challenge run by the University of Chicago's Polsky Center for Entrepreneurship and Innovation. "Roughly 110 teams entered," says Giger. "Twenty-some reached the next step, which was an oral presentation about the science and the business. And then nine reached the finals, including us."
Getting FDA clearance
Immediately after the Challenge, the team founded Quantitative Insights to incubate and commercialize QuantX. Quantitative Insights received mentoring and other support from the Polsky Center. They applied for a de novo marketing authorization from the FDA; if successful, it would grant Quantx a first-of-its-kind status. "We had to conduct an extensive clinical study," says Giger. "We analyzed a range of cancers and benign lesions, and conducted an evaluation with a range of radiologists — they were all certified to read breast MRIs, but some were in practice for a long time, some were new, some were academic, some were in private practice."
In the summer of 2017, Quantitative Insights received that de novo status, so QuantX was cleared for the next hurdle: clinical installation. "You learn about many aspects of clinical translation at this stage," says Giger. "You have to implement cybersecurity, you have to make sure the software can safely sit on a clinical network, it's a step far beyond conducting lab-based research. Even with an FDA-cleared product, QuantX was not quite ready to be dropped into the clinical arena because of the additional aspects of, "Does the software communicate appropriately on the clinical network? Is it compatible? If there are errors in the image data, will the system crash? What is disaster recovery? All that had to be learned."

The success of the development is continuing with Paragon Biosciences' acquisition of QuantX. "It's great," says Giger, "because the new company, Qlarity Imaging, has the funding and resources to extend the product to go beyond just breast MRIs — we want to be able to use this technology on mammograms and ultrasounds; we want to go beyond breast cancer, to lung cancer and prostate cancer." As an academic investigator, she adds, "you spend your life researching and developing innovative methods; you want it out there in the public for the benefit of others."
That said, she appreciates that reaching that goal required the aforementioned time, patience, and due diligence. "I used to ask myself, ‘Why can't I just give my research away? I want everyone to have it.' But I have learned that research software given away is not actually ready for clinical practice; it needs to be rigorously tested and proven to be safe and effective, and then converted into a commercial product capable of being safely installed on a clinical network. Finding a company with the economic incentive to advance the product through the FDA and complete commercialization is critical."
An interactive SPIE community
Access to engaged networking, dynamic collaboration, and bigger-picture views, she says, is also imperative. It's important to identify your community and expand outwards. "I grew up at SPIE Medical Imaging," says Giger. "I've been attending that conference since I was a student in the early 80's, and the reason I like it so much is because it spans all of imaging. We have the physics of imaging conference, we have image processing, we have computer-aided diagnosis that includes machine learning/deep learning, we have functional imaging, we have digital pathology, we even have image perception, and metrology. It's not just about image analysis, it's about everything!"
Having that big-picture access has led to Giger's own success, and she's extended that opportunity to others. "For example, as a conference chair, I brought in radiologists because many people developing computer-aided detection and computer-aided diagnosis algorithms did not have access to radiologists, never sat with them, never watched how they interpreted medical images. So around 20 years ago, we invited radiologists to SPIE Medical Imaging and, in one of the workshops, we asked them to interpret various medical images in front of all the attendees, who were able to observe the radiologists' eye-brain thought processes."
Giger is looking forward to the 2020 SPIE Medical Imaging meeting where her colleagues are planning an interactive exploration of digital pathology. "Many hospitals have what is referred to as a tumor board, where various health professionals meet together — the radiologist, the pathologist, the oncologist - and review a case at multiple scales: they look at the mammogram, the MRI, the histopathology as well as the clinical data. "Such a tumor board will be mimicked at SPIE MI with multiple medical specialists on a panel, sharing their insights and views." And, ideally, sharing a big-picture vision that will take other researchers successfully into the future.
| Enjoy this article? Get similar news in your inbox |
|



