Clear-sighted neural network counts corneal cells
The microscope uses a combination of optical coherence tomography, a liquid lens, and a microelectromechanical system mirror to provide beautifully sharp corneal images that help technicians assess the quality of the donated cornea. When combined with a neural network, automated cell counting prior to corneal translplant may be possible.
According the National Institutes of Health, US surgeons performed 47,000 corneal transplants in 2014. Yet, before the procedure, each donated cornea has to be inspected to verify that it's suitable for transplantation, and both the inspection and the surgery have to take place in a short window of time before the donated tissue begins to die. This medical production line is even more impressive when you realize that cornea inspection involves cell counting, which is a task that is performed manually by trained technicians.
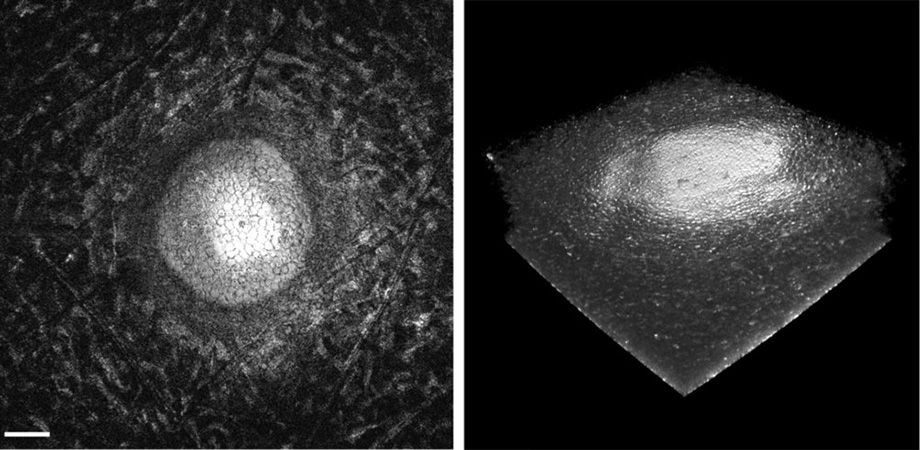
Cornea inspection presents a challenge: you need to check the health of the whole cornea (several cubic millimeters), yet you also need to be able to see individual cells to check the cell density, which indicates the health and clarity of the cornea. Just to add to the challenge, it is preferable to do the inspection without opening the container holding the cornea to avoid damaging it. Not only does this mean working through the transparent lid of the container, it also means that the distance from the optical system to the cornea is not precisely known and cannot be finely manipulated.
A Rochester based startup, LighTopTech, in collaboration with University of Rochester, is working towards adapting their innovative microscope design to use an automated cell-counting technique that comes close to meeting all of these challenges. CEO and co-founder Cristina Canavesi presented the novel approach at SPIE Photonics West BIOS 2019.
A cornucopia of technologies
The microscope uses a combination of optical coherence tomography (OCT), a liquid lens, and a microelectromechanical system (MEMS) mirror to provide beautifully sharp corneal images that help technicians assess the quality of the donated cornea. Then, because cell-counting is tiresome and highly subjective, the researchers developed a machine learning algorithm to automate the process.
OCT uses the wave nature of light to provide depth information. Light from an LED is split into two. Half the light is directed into the cornea, where a small fraction is reflected back into the microscope by the cell walls of the corneal tissue. The other half of the light is used as a reference: it travels a known path before returning to the point where the light was split. The two returning light fields are mixed to create an interferogram that reveals the depth in the cornea from which light was scattered.
The next step is to vary the focal length of the microscope objective so that a series of sharply focused 3D images can be stitched together. To accomplish this, the bioinspired microscope utilizes a liquid lens to squeeze and relax the fluid, changing both the curvature and focal length of the lens.
The change in focus accomplished by the liquid lens is quite significant in this case, because the cornea, though thin, is curved. To obtain sharp images over the entire thickness of the cornea at the edges and in the center, the focal point of the objective has to scan about 2.5 mm. At each focal depth, the MEMS mirror then scans the focal location laterally to obtain a large field of view.
Clear-sighted neural network
The next intriguing step to their approach moves us toward a future when the task of cell-counting could be automated. Since the endothelium, a single layer of cells whose density is an important metric related to corneal health, is curved, it is necessary to perform image processing in order to produce a clear view of the endothelial cells in a single layer. The cells can then be counted in an automated fashion.
The researchers developed a fairly straightforward polynomial fit function to remove curvature, followed by a sophisticated neural network to count cells. To test and train the neural network, the researchers imaged six corneas in various locations to get 180 images (30 unique locations, imaged six times). The cells in these images were manually counted-although manual in this case still means a lot of image-processing tricks to speed the process.

Corneal endothelial surface, extracted from the GDOCM 3D image over a 1 mm x 1 mm field of view through a custom flattening algorithm.
120 images were used to train the neural network, while the remaining 60 were used to test the network. Interestingly, the neural network seems to be much more sensitive to cells near the edge of the image compared to the manual counting method. This has two consequences: it may mean that automated cell counts become more representative of true cell density, but it certainly also means that comparing between cell count methods will not be straightforward.
What is clear, though, is that we are nearing a point where cornea analysis might become fully automated. This is thanks to an innovative women-led startup taking advantage of a confluence of various optical technologies: OCT-which is already used to help analyze cornea- MEMS optical components, and liquid lenses that are now of sufficient quality to be used in microscopes.
Watch the presentation on the SPIE Digital Library.
Canavesi acknowledges funding from the National Institutes of Health under Grant No. 1R43EY028827-01, and National Science Foundation under Grant No. IIP-1534701
| Enjoy this article? Get similar news in your inbox |
|



